Pipeline | IFx-2.0
IFx-2.0 Personalized Innate Immune Agonist
IFx-2.0 personalized innate immune agonist involves a simple injection into a patient’s tumor of a small amount of pDNA that encodes for an immunogenic bacterial protein which gets expressed on the surface of the patient’s tumor. In doing so, IFx-2.0 is designed to harness the power of the patient’s innate immune response, which has evolved over time to be conserved to detect foreign pathogens like bacterial proteins. By making the surface of a tumor look like a bacterium, IFx-2.0 is designed to use the tumor itself as the source of foreign neoantigens to prime and initiate an innate immune response against the tumor irrespective of whether the tumor escaped immune recognition prior to IFx-2.0 administration.
IFx-2.0 Summary Phase 1/1b Clinical Trial Results
Produced high rates of durable systemic objective responses across multiple cutaneous malignancies including in advanced or metastatic Merkel Cell Carcinoma, cutaneous Squamous Cell Carcinoma and Melanoma that exhibited primary resistance to checkpoint inhibitor therapy
Excellent safety profile when used as adjunctive therapy with a checkpoint inhibitor
Entering single Phase 3 registration directed pivotal trial to be conducted under Accelerated Approval pathway for first line treatment of advanced or metastatic Merkel Cell Carcinoma when used as adjunctive therapy with Keytruda® (pembrolizumab)
Demonstrated Ability to Overcome CPI Resistance Across Multiple Tumor Types in Phase 1/1b Studies
TuHURA has completed enrollment in a multicenter Phase 1b dose and schedule finding trial for the company’s IFx-2.0 innate immune agonist candidate in patients with advanced Merkel Cell Carcinoma (MCC) or cutaneous Squamous Cell Carcinoma (cSCC). This study follows a two-stage design with a primary goal to assess the safety and feasibility of repeated dosing schemas of IFx-2.0. In the first stage (exposure escalation), a 3+3 trial design was utilized to assess the safety of repeated weekly intratumoral vaccinations using a fixed dose of IFx-2.0 (Cohort 1 = single dose; Cohort 2 = 2 doses, 1 week apart; Cohort 3 = 3 doses, weekly for 3 weeks). An expansion stage was conducted to increase the total study sample size to 20. As of August 15, 2023, a total of 22 patients have been enrolled.
A total of 9 patients were treated across the 3 dosing cohorts in the first stage of the study. Of the 9 enrolled patients; 6 had advanced MCC and 3 patients had advanced cSCC. Five of the 6 patients with MCC and 2 of the 3 with advanced cSCC exhibited primary resistance to checkpoint inhibitor therapy. Following completion of IFx-2.0 protocol therapy, 5 patients with MCC and 2 patients with cSCC who failed first time therapy with a CPI were re-treated with anti-PD(L)-1 checkpoint inhibitor monotherapy as the immediate post-protocol treatment: pembrolizumab (3) or avelumab (2) in Merkel cell and cemiplimab (2) in Squamous cell. Four of 5 patients with Merkel cell and 1 of 2 patients with Squamous cell, or 5 of 7 total (71%), experienced an objective response to checkpoint inhibitor rechallenge with duration of response ongoing in 4 patients (7+, 8+, 9+, 20+ months) and one response lasting 23 months. All patients with Merkel cell with post-protocol response to anti-PD(L)-1 checkpoint inhibitor therapy had previously experienced progression to this same drug class prior to treatment on protocol. Post-protocol checkpoint inhibitor rechallenge treatment responses among patients with advanced MCC and cutaneous Squamous cell carcinoma are shown in the tables below.
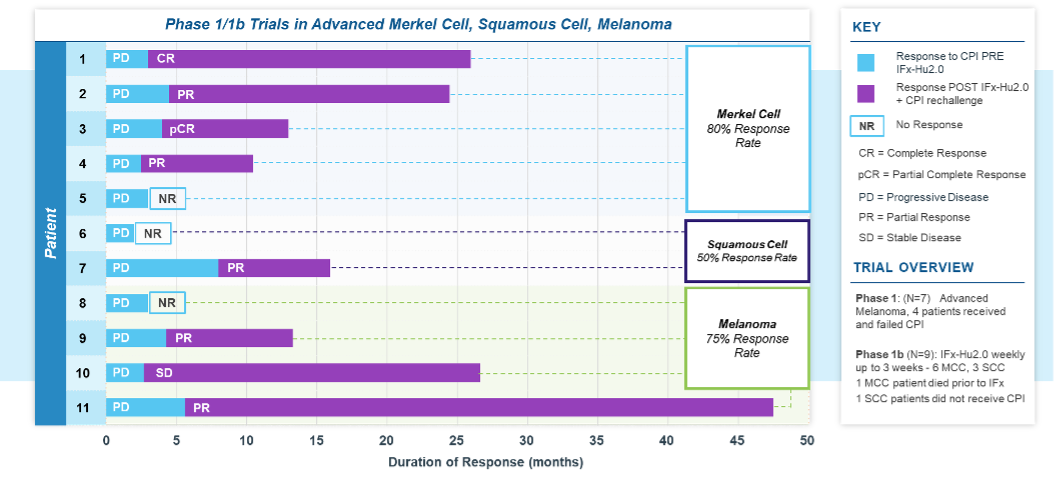
Importantly, IFx-2.0 is not an intratumoral therapy like oncolytic viral therapies whose anti-tumor activity is limited to accessible, injected lesions in limited stages of cancer. In contrast, IFx-2.0’s mechanism of action is to prime and activate an innate immune response in injected lesions which leads to systemic activation of an adaptive immune response. TuHURA chose to examine IFx-2.0 in cutaneous malignancies because human skin has a high density of dendritic cells (DCs) which are very efficient in presenting foreign antigens to immune cells. Local injection of IFx-2.0 into cutaneous lesion(s) has resulted in immune cell infiltration, tumor neoepitope presentation, in the context of MHC I and MHC II, to naïve B and T cells followed by activation of tumor specific B and T cells. The immune response is not restricted to just injected lesions but rather a systemic immune response is generated as demonstrated by production of tumor specific IgM and IgG antibodies in the plasma of patients post IFx-2.0 administration and prior to checkpoint inhibitor therapy rechallenge. For example, following IFx-2.0 patients with MCC generated both IgM and IgG antibodies against major and minor polyoma viral oncoproteins prior to CPI rechallenge. The breadth of antibody production correlated with clinical responses observed following CPI rechallenge.
IFx-2.0 Overcomes 10 Resistance to Keytruda® (pembrolizumab) in MCC
Demonstrates Systemic Activation of Immune Response (Abscopal Effect)
IFx-2.0 Weekly x3 – Followed by Keytruda® (pembrolizumab)
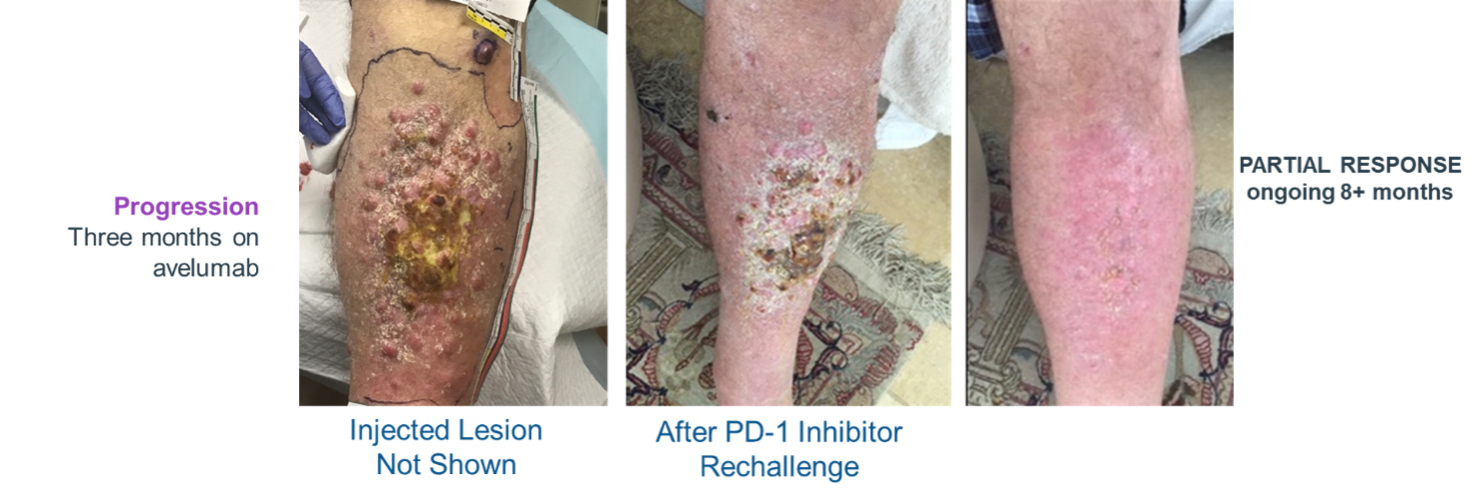
Overcomes 10 Resistance to Keytruda® (pembrolizumab)
in MCC
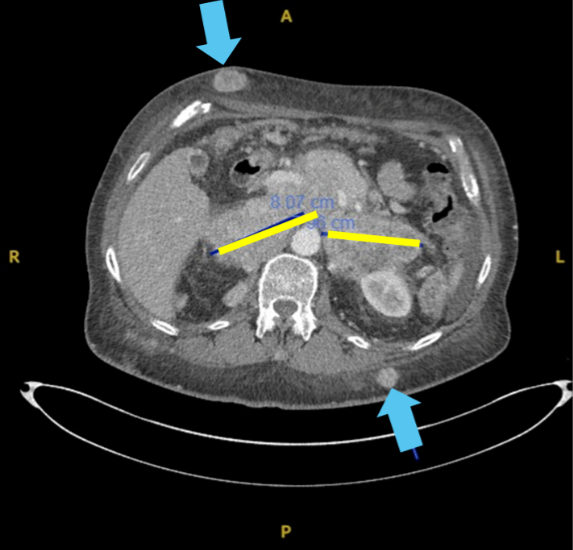
Large bulky retroperitoneal nodes (yellow)
Received IFx2.0 weekly x 2 in dermal lesions (blue arrows)
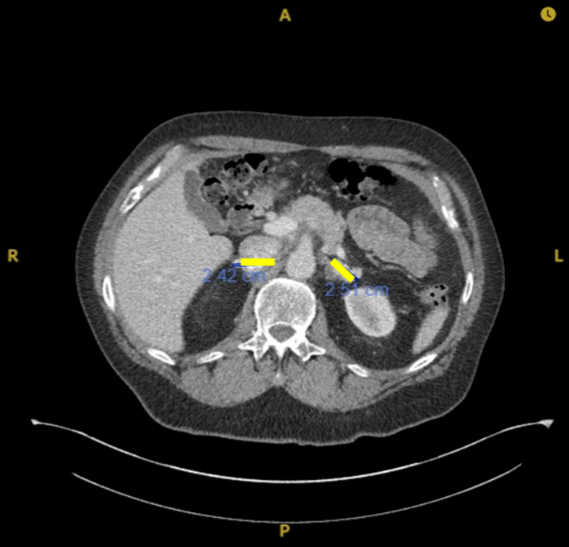
Post IFx-2.0 rechallenged with avelumab
Complete disappearance of subcutaneous nodules
80% reduction (Partial Response) in abdominal masses
Responses are ongoing 20+ months
Initially Targeting Advanced Merkel Cell Carcinoma (MCC)
Aggressive form of skin cancer, driven by polyoma viral oncogene with 5-year survival <25% for advanced disease
Up to 50% of Patients Have Primary Resistance and Don’t Respond to 1st Line Keytruda® (pembrolizumab)1
US incidence ~2,500/year, increasing 30% by 20251
Unmet Medical Need – up to 50% of Patients Have Primary Resistance and don’t respond to 1st line Keytruda® (pembrolizumab)2
- Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457-463.e2. doi:10.1016/j.jaad.2017.10.028
- N Engl J Med 2016; 374:2542-2552 DOI: 10.1056/NEJMoa1603702
