Pipeline | IFx-3.0
IFx-3.0 Personalized Innate Immune Agonist Delivered Intravenously
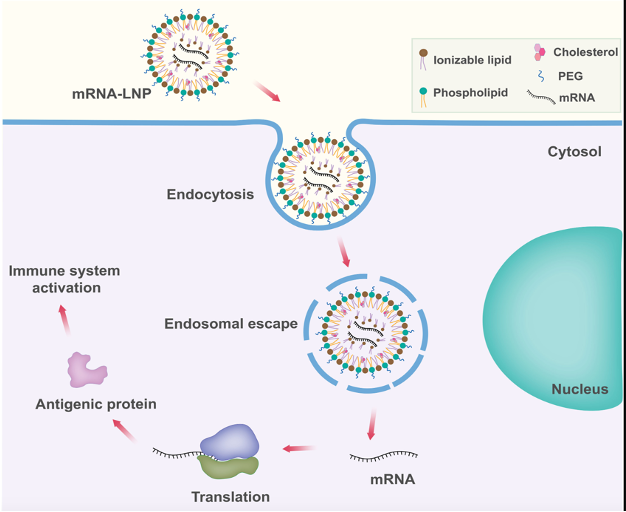
IFx-3.0 is leveraging the clinical validation of our leading IFx-2.0 program and is being developed for intravenous delivery of our proprietary emm55 mRNA targeting CD22, which is overexpressed on cancerous B-cells.
emm55 mRNA codes for the same highly immunogenic bacterial protein utilized in our IFx-2.0 program. We are able to have tumor cells produce the emm55 bacterial protein by using a proprietary codon-optimized mRNA necessary to improve the efficiency of translation. Mechanistically similar to our pDNA IFx-2.0 innate immune agonist, delivery of the mRNA causes this bacterial protein to be expressed on the surface of the tumor cells, priming the immune system to target and attack tumor cells by making them look like bacteria.
While our IFx-2.0 has been utilized for tumors accessible by intratumoral injection, developing a systemically targetable mRNA innate immune agonist allows us to expand the application of our personalized innate immune agonist to blood related cancers like leukemia or lymphoma or multiple myeloma. In aggressive lymphoma, malignant B cells populate the bone marrow, lymph nodes, spleen and blood representing large tumor burden that, if transfected with our mRNA innate immune agonist, could result in the activation of a more robust systemic immune response.
We are developing a proprietary single chain variable fragment (scFv) that has a high affinity and avidity for the CD22 receptor which is overexpressed on B cell malignancies like aggressive lymphomas. CD22 is an ideal target as it is internalized when bound to a ligand bringing into the endosome our emm55 mRNA. Since aggressive lymphomas have a high proliferative index, we would expect high efficiency of translation to the emm55 protein and expression on the surface of the CD22 expressing malignant B cells. Our lead IFx-3.0 gene therapy candidate will utilize lipid nanoparticle packaged emm55 mRNA coupled to our proprietary CD22 targeting scFv. We expect to begin IND enabling studies in the 2nd half of 2024.
Program Highlights
-
First CD22 tumor targeted mRNA innate immune agonist
-
Developed proprietary CD22 targeting antibody fragment (scFv) coupled to mRNA loaded lipid nanoparticle
-
CD22 overexpressed on malignant B cells allowing IFx-3.0 mRNA payload to be selectively targeted to and expressed in cancerous B cells
-
Expands innate immune agonist to blood related cancers